Source: Nature
Abstract
To determine whether photobiomodulation (PBM) therapy can retard ocular axial length (AL) in children with myopia. A randomized controlled clinical trial was conducted on two consecutive cohorts of 50 eligible children aged 8–12 years with ≤ − 0.75 Diopter (D) of spherical equivalent refraction (SER). Participants were randomly assigned to the intervention group (n = 25) and treated with PBM therapy or the control group (n = 25) and treated with single vision spectacles only. At the 12-month follow-up, the changes in AL and cycloplegic SER from baseline were both compared between the two groups. In addition, the subfoveal choroidal thickness (SFChT), anterior chamber depth (ACD), and central corneal refractive power (CCP) were analysed at the 3-, 6-, 9-, and 12-month follow-ups, respectively. Among the 50 children, 78% were included at the final follow-up, with a mean age of 9.7 ± 1.5 years and a mean SER of − 2.56 ± 1.70. The mean difference in AL growth between the two groups at 12 months was 0.50 mm (PBM vs. Control, − 0.02 mm ± 0.11 vs. 0.48 mm ± 0.16, P < 0.001), and the mean difference in cycloplegic SER at 12 months was + 1.25 D (PBM vs. Control, + 0.28 D ± 0.26 vs. − 0.97 D ± 0.25, P < 0.001). There were no significant differences in any of the other parameters (including SFChT, ACD, and CCP) between the two groups at any time point. PBM therapy is an effective intervention for slightly decreasing the AL to control myopia in children.
Trial registration: Chinese Clinical Trial Registration Number: ChiCTR2100043619. Registered on 23/02/2021; prospectively registered. http://www.chictr.org.cn/showproj.aspx?proj=121302.
Introduction
Sufficient outdoor time is a widely accepted method for preventing myopia1. High levels of ambient lighting could retard form-deprivation myopia in monkeys2. The change in ocular axial length (AL) involves a tremendous change in the light environment, which can be considered to play an important role in the onset and progression of myopia. The difference between indoor and outdoor light environments, such as the intensity and wavelength of electronic lighting equipment, may be a cue for myopia control as environmental interventions.
Fortunately, some clinical trials have discovered a novel and intriguing intervention—which has many different names, e.g., low-level laser therapy (LLLT)3, low-intensity, long-wavelength red light4, or repeated low-level red light (RLRL)5—to control myopia in children with approximately two 3-min sessions of therapy every day3,4,5,6,7,8. Those studies have suggested that two sessions of therapy with repeated low-intensity, single-wavelength (650 nm) red light for a total of 6 min daily could significantly retard AL) growth. Moreover, the recently reported maximum decrease in AL was 1.07 mm, which was observed in a 5-year-old high myopia patient (from baseline − 8.50 D to − 5.50 D after 10 months of red light therapy) on social media9.
The first well-designed randomized controlled study also reported that this special red light therapy could shorten the AL to a mean value of − 0.06 mm (74 children)3 after 6 months compared to the single-vision spectacles (SVS) group, who exhibited a mean AL increase of 0.23 mm (74 children), as well as the orthokeratology group, who exhibited a mean AL increase of 0.06 mm (81 children). Thus, all of the above findings on decreased ALs were quite opposite to the traditional concept that the elongated eyeball was irreversible. Although some evidence has demonstrated that PBM therapy only slows AL growth5 with a rebound effect6,7, it could be a promising pantheon for light-based therapeutic intervention.
Additional evidence regarding the effect of PBM therapy on shortening the AL in myopia came from the authorized PCT patent WO2020244675A112 with a disclosure date of 2020. In the patent, a similar phenomenon was observed, including a decrease in AL for 84 myopic children (6–23 years old) for several months (one child underwent therapy for 3 months, the other 83 children underwent therapy for 6 months) after PBM therapy twice a day.
Previously, we published a retrospective cohort study4 of 105 myopic children, and we found that their AL was decreased by − 0.06 ± 0.19 mm for 9 months after PBM therapy. To overcome the limitations of previous studies that lacked control groups, the high possibility of long-term observation of the tendency of AL growth in children with myopia, and AL circadian rhythm fluctuations10, we conducted the current prospective study to examine whether the AL of individuals with myopia might be decreased extent after 12 months of repeated PBM treatment twice a day.
Methods
Study design
This 12-month clinical trial was designed to be a randomized, controlled, parallel study with follow-up visits every 3 months. Potentially eligible children were recruited from the hospital after a screening visit.
The trial was designed to begin in February 2021 and end in June 2023. All subjects were enrolled at the Optometry Center of Ningbo Eye Hospital. Our study and protocol conformed to the principles of the Declaration of Helsinki and were approved by the ethical committee of Ningbo Eye Hospital (Number: 2020xjx-012).
Eligibility criteria
The inclusion criteria were as follows: (1) the subject’s guardian agreed to participate in the study and signed a written informed consent form; if the subject could express his or her willingness to participate in the trial, the subject’s consent was also obtained; (2) age 3–16 years old (including the boundary values); there were no restrictions regarding gender; (3) the cycloplegic refraction with either spherical equivalent refractive (SER) was ≤ − 0.50 D ~ − 8.00 D (including the boundary values), and the total astigmatism was ≤ 2.50; (4) spectacle-corrected monocular VA was 0.0 logMAR or better; and (5) willing to wear SVS throughout the trial.
The exclusion criteria were as follows: (1) patients who intended to use atropine (including 1% high concentration or 0.01% low concentration); (2) patients wearing peripheral defocus spectacles or duo-focal soft contact lenses; (3) patients with eye diseases such as glaucoma or retinal lesions; (4) optic media lesions (e.g., central thick corneal scars, cataract); (5) patients with optic nerve dysfunction; (6) patients with amblyopia; (7) research physicians determined that the subject was not eligible for other reasons. After screening, the participants were selected based on professional inquiry and baseline examination. The participants and their parents or legal guardians were informed about the benefits and risks of this study before providing signed informed consent on behalf of their children.
Interventions and visits
All spectacle lenses were made for single focus with full correction for each subject as the first intervention throughout the whole procedure for both groups. PBM therapy was performed with a low-intensity laser (LD-A, Jilin Londa Optoelectronics Technology, Jilin, China) with an irradiance of 0.35 ± 0.02 mW/cm2, a wavelength of 650 nm ± 10 nm, and illumination of approximately 400 lux on average.
During the baseline visit, eligibility was evaluated, and baseline measures were conducted. The dates of all subsequent visits were determined based on completion of the baseline examinations. Cycloplegic refraction using an automatic refractometer (Topcon KR-800, Topcon Corporation, Tokyo, Japan) and 100% subjective trial lenses were recorded at baseline and 12 months, respectively. Cycloplegia was achieved using 3 drops of 1% cyclopentolate hydrochloride eyedrop (CYCLOGYL, Alcon Laboratories, Inc., USA), and the spherical equivalent refraction (SER) was determined (obtained with the following formula: SER = spherical diopter + astigmatism/2). The follow-ups were conducted at 3 months, 6 months, 9 months and 12 months. The values of AL (IOLMaster 500, Carl Zeiss Meditec AG, Germany), CCP (Pentacam 70700, Oculus, Inc., Germany), SFChT (Spectralis OCT, Heidelberg Engineering GmbH, Germany) and ACD (Pentacam 70700, Oculus, Inc., Germany) were all recorded and evaluated at each visit in addition to the baseline visit.
Other ophthalmologic examinations included slit-lamp examination (HS-5000(HLG), Huvitz Co. Ltd, Korea), noncontact tonometry (Topcon CT-80, Topcon Inc., Japan), and fundus scan with optical coherent tomography (Spectralis OCT, Heidelberg Engineering GmbH, Germany).
Evaluated parameters
The primary outcome variables were changes in AL and SER compared to baseline. SER (sphere plus half cylinder) from the pattern of five measurements was measured at least 30 min after instillation of 2 drops of 1% cyclopentolate administered every 5 min. AL was measured by calculating the average of five measurements obtained from the same IOLMaster. The secondary outcome variables included SFChT (Spectralis OCT, Herdingberg, Germany), ACD (from IOLmaster 500) and CCP (from Pentacam). PBM therapy was administered twice a day for 3 min per session with an interval of ≥ 4 h between sessions based on the self-report of participants or their supervision. We also evaluated the subjects’ social context, such as schools, and lifestyle (e.g., outdoor time) via questionnaires.
Due to the small sample size, we aimed to avoid the influence of age on different growth rates of AL. Therefore, the eligibility criteria for age were set from 8 to 12 years old. In addition, we also removed the limitation on baseline AL ≥ 24.40 mm because the myopia suppression effect is unknown for axial lengths greater than 24.40 mm.
Sample size calculation
Power Analysis and Sample Size Software (PASS 2022) (NCSS, LLC. Kaysville, Utah, USA) was used to determine that the minimum sample size was 24. A previous study found that the rate of AL change was approximately 0.25 mm/year (0.13 mm/year vs. 0.38 mm/year) slower in participants treated with repeated low-level red light than in controls5. The mean AL progression was 0.13 mm and 0.38 mm with a standard deviation (SD) of 0.15 mm after 1 year in the control group based on previous findings3,4. This was based on a two-sided statistical test with a 1% type I error threshold, 90% power and a 20% drop-out rate.
Randomization
Eligible participants enrolled in the study were assigned to receive single-vision spectacles (SVS) only or photobiomodulation (PBM) therapy with SVS at a 1:1 ratio using a randomization sequence produced by Excel. The mechanism used to implement the random allocation sequence was sequentially numbered containers by random envelopes. The enrolled subjects were randomly divided into two groups based on random numbers placed in envelopes. The random numbers were generated by Excel, and the aim was to achieve balanced treatment groups based on their baseline right-eye refractive error, mean age and gender. After all 50 participants were enrolled, the random envelopes were opened to indicate the group assignment. Researchers who were assessing outcomes and performers (for cycloplegic autorefraction and AL measurements) were still blinded to group allocation, but participants and care providers were not blinded after the random envelopes were opened.
Adverse events
Those who received at least one session of therapy were analysed for safety. At each follow-up visit, the participants were asked about the symptoms and signs, including ocular symptoms (such as glare, dazzling, afterimages and flash blindness) and systemic adverse effects (such as headache or dizziness). Other information included the best corrected vision acuity (BCVA), anterior segment with slit-lamps and fundus with OCT. Additionally, each participant was also asked to report the reversible time of vision loss due to glare, dazzling, afterimages or flash blindness. Adverse events were reported based on interviews at the 12-month follow-up visit and were scored according to the common terminology standard for adverse events (CTCAE) version 5.0.
Statistical analysis
The data were analysed by IBM SPSS statistical software 22.0 (IBM Co., Armonk, USA). Continuous variables were analysed with a two-sample independent t test after being evaluated for normality using the Kolmogorov‒Smirnov test. If the data conformed to a normal distribution, the mean ± standard deviation was reported. Fisher’s exact test or the χ2 test was conducted to compare the gender ratio between the two groups. ANOVA was used to compare the means between the baseline and follow-up visits. If the data were not normally distributed, the median (interquartile range) or median (95% confidence interval, 95% CI) was reported, and nonparametric tests were performed. A P value < 0.05 indicated significant differences. Only the data for the right eye were used.
The independent samples t test was used to analyse the changes in AL (as the primary endpoint), ACD, SFChT, and CCP at the 3-, 6-, 9-, and 12-month follow-ups as well as changes in cycloplegic refraction at the 12-month follow-up.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of Ningbo Eye Hospital (No: 2020xjx-012).
Conference presentation
This paper was presented at the conference of the 26th Congress of Chinese Ophthalmological Society (CCOS) 2022 and the World Ophthalmology Congress (WOC) 2022 online meeting.
Results
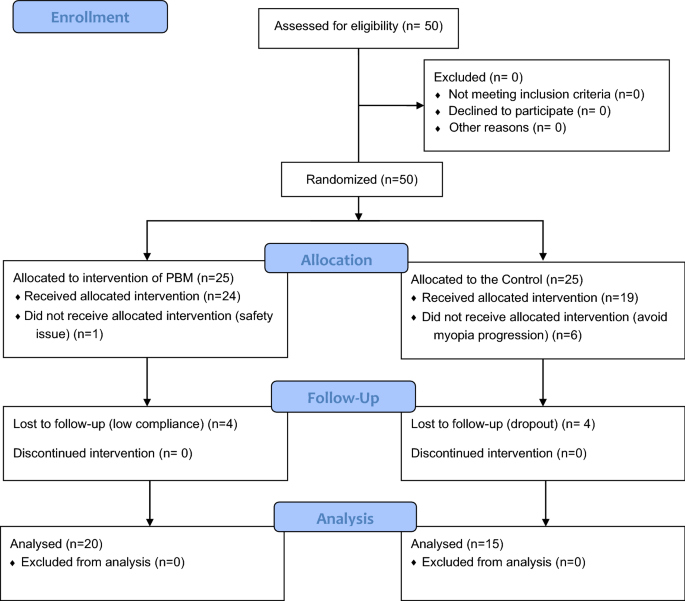
From February to May 2021, 50 eligible subjects signed written informed consent forms before randomization, 43 subjects (24 in the PBM therapy group and 19 in the control group) completed the baseline visit, and 35 subjects completed the final 12-month follow-up visit (20 in the PBM group and 15 in the control group). The details of the participants are shown in Tables 1, 2 and 3. The flow diagram of the sample size and reasons for dropout are shown in Fig. 1.
The characteristics of the baseline data are shown in Table 1. The total proportion of male participants was 61%, with a mean age of 9.7 ± 1.5 years. The cycloplegic SER of the right eyes ranged from − 0.75 to − 8.00 D with a mean value of − 2.56 ± 1.70 The mean AL, SER, ACD, CCP and SFChT did not differ based on any demographic variables except for age. The average age of the PBM therapy group was 1 year significantly older than that of the control group. The ocular characteristics (including AL, SER, ACD, CCP and SFChT) and sex were well balanced in both groups (Table 1). At baseline, no statistically significant difference was detected between the right and left eyes.
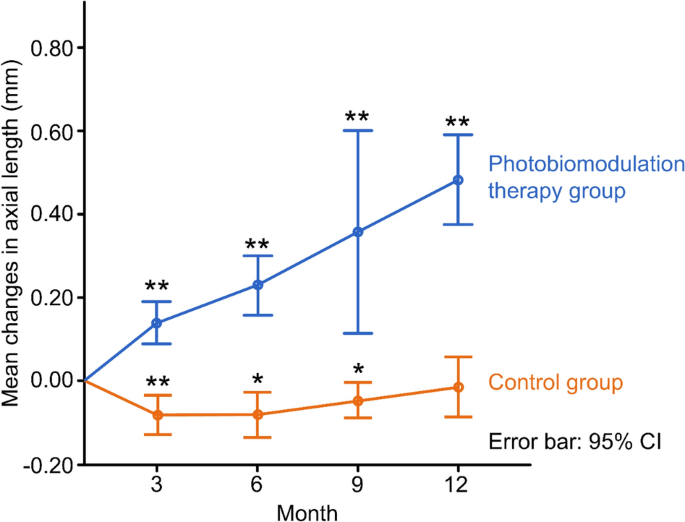
The mean change in AL decreased slightly in the PBM therapy group from baseline to the 12-month follow-up. In contrast, the mean change increased in the control group. When compared with the control group, the change in AL of the PBM group at each follow-up visit (3, 6, 9, and 12 months) was significantly better (− 0.09 ± 0.07 vs. 0.14 ± 0.06, P < 0.0001; − 0.07 ± 0.09 vs. 0.23 ± 0.11, P = 0.0001; − 0.05 ± 0.06 vs. 0.36 ± 0.15, P = 0.007; − 0.02 ± 0.11 vs. 0.48 ± 0.16, P < 0.0001, respectively), as shown in Table 2 and Fig. 2.
Changes of axial length of two groups at 3-, 6-, 9-, 12-month follow-up, respectively. ** and *represent P < 0.001 and P < 0.01 comparing the data of each follow-up with that of no change from baseline.
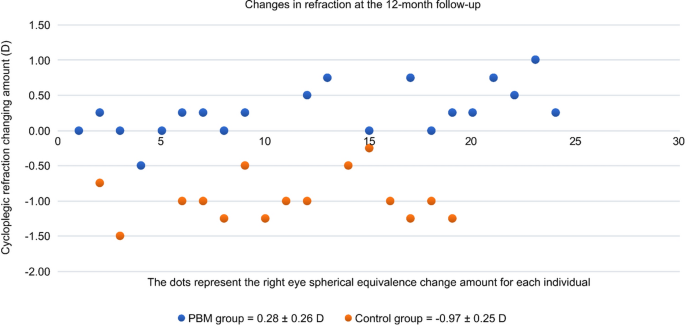
Accordingly, the mean change in cycloplegic SER at 12 months from baseline was also significantly better in the PBM therapy group than in the control group (+ 0.26 ± 0.27 vs. − 0.97 ± 0.25, respectively, P < 0.0001). The mean SER value of the PBM therapy group decreased by approximately + 0.26 D, while the mean myopia of the control group progressed by − 0.97 D during the study (Fig. 3). The positive value (+ 0.26 D) of the mean SER change perfectly matched the negative change in the mean AL (− 0.02 mm), which explained the stabilization of myopia progression due to PBM therapy during the 12 months. The axial length of the control group increased, and the average calculated growth rate was − 2.02 D/mm (− 0.97 D/0.48 mm) during the 12 months.
Changes in refraction individually at 12-month visit. PBM photobiomodulation therapy, Control single vision spectacles only.
There were no significant changes in any of the other variables, including CCP, ACD, and SFChT, between groups at any visit (Table 2). The myopia control rate and the dropout rate were calculated in terms of the AL and cycloplegic SER at the 12-month follow-up in both groups, and the results are shown in Table 3. The control rate was defined as an AL or SER at follow-up that was lower than the value at baseline. Similarly, the control rate of the PBM therapy group was also significantly higher than that of the control group. Specifically, the percentages of individuals who achieved AL and SER control were 60% and 90%, respectively, in the PBM group, while the percentages of individuals who attained AL and SE control were 0% and 0% in the control group. In addition, the withdrawal rate of the control group (40%) was twice as high as that of the PBM group (20%). Furthermore, neither social context, such as economic situations mainly by family income, nor lifestyle, such as near work and outdoor time, were significantly different between the two groups (Table 4).
The most common adverse event was reversible vision loss, lasting approximately 2.1 ± 0.7 min (n = 23) after each PBM therapy due to flash blindness or glare with afterimage. In addition, afterimage, which lasted a little bit longer than the reversible vision loss—an average of 3.2 ± 1.2 min (calculated from the moment immediately lighting off of 650 nm PBM therapy)—was positive afterimage, i.e., central scotoma with different shapes in bright pink from the subjects after each PBM therapy (except the condition of closing the eyes). According to CTCAE (version 5.0), all cases were reversible and were all considered grade 1 eye disorders. All the above symptoms were subjective without objective abnormal findings from anterior or posterior ocular segments. There were no systemic adverse effects (such as headache or dizziness), severe adverse events, or other adverse events related to grades of ocular diseases: Grade 2–5 of CTCAE (version 5.0). Additionally, there was no dry eye, cataract, keratitis, night blindness, photophobia or any other permanent visual impairment.
There was one subject whose supervisor refused to join PBM therapy after randomization due to concern about safety issues, including the abovementioned temporary and reversible vision loss and afterimage phenomena. None of the reasons for dropout during follow-up in the PBM group were related to adverse events. An explanation was given for the different dropout rates between the control and PBM groups at baseline (6 vs. 3 dropouts, respectively) and at the 12-month follow-up (4 vs. 2 dropouts, respectively) (Fig. 1). After randomization, in the absence of additional intervention, the main reason for dropout in the control group was unacceptable (only SVS), whereas the main reason for dropout in the PBM group was understandable, as it was due to the uncertain harm of this new therapy. The COVID-19 pandemic was the main reason for the other missing data, especially at the 9-month visit, and both groups of patients were unable to visit regularly at that time.
Discussion
As shown in Table 5, those results were consistent with our previous report and the studies of Xiong et al.3,4,20. It was shown that myopia among children could be controlled or reversed to some extent by PBM therapy, resulting in a mean decrease in the AL. Compared with baseline, this algorithm achieved 90% and 60% decreases in myopia based on SER and AL changes, respectively, instead of the values calculated based on the control group. However, because of the negative changes in AL and positive changes in SER, if the efficacy algorithm was based on the values from the control group with a formula of control rate = ChangeChangeofofPBMPBMgroupgroup−ChangeChangeofofcontrolcontrolgroupgroupChangeChangeofofcontrolcontrolgroupgroup�ℎ�����ℎ������������������������−�ℎ�����ℎ���������������������������������ℎ�����ℎ��������������������������������*100%, both AL (103%) and SER (127%) control rates were > 100%. Jiang Y et al. used the same algorithm and observed yearly AL and SER control rates of 66% and 75%, respectively. The difference was probably due to their frequent administration of 5 times a day, including their reported low compliance.
As one of the most effective interventions compared with other interventions12,13,14,15,16,17,18,20,21, we found evidence supporting our hypothesis that the progression of myopia as measured by the AL is reversible using PBM therapy twice a day.
All the negative mean changes in AL from the study group at 3, 6, 9, and 12 months (− 0.09 ± 0.07 mm, − 0.07 ± 0.09 mm, − 0.05 ± 0.06 mm and − 0.02 ± 0.11 mm) demonstrated that PBM could retard or control increases in AL. In contrast, all the positive mean changes in AL from the control group at each visit (0.14 ± 0.06 mm, 0.23 ± 0.11 mm, 0.36 ± 0.15 mm, 0.48 ± 0.16 mm at 3, 6, 9, and 12 months, respectively) revealed a strong natural tendency towards increased AL among primary school-age children. Although the age of the control group was significantly younger by 1 year, the decrease in the mean values of AL in the PBM therapy group also indicated that the administration with a frequency of twice daily in our trial was better than the frequency of five times a week in Jiang Y et al.’s study with an mean AL increase of 0.13 mm per year5. In addition, in past prospective myopic interventions, almost no negative change in mean AL per year was reported, and the only comparable effect was 1% atropine treatment. As reported by Yi S et al. the negative average change in the mean AL was − 0.03 ± 0.30 mm11. Balancing the pros and cons, PBM therapy had much less photophobia, much shorter blurred vision time and much fewer other AEs than 1% atropine therapy11 in our study or repeated low-level red light (RLRL) therapy5,7. The other optical interventions are partly shown in Table 5, such as Misight myopia control disposal soft contact lenses13,14, different low concentrations of atropine eyedrops12,15,16, innovative spectacle lenses with D.I.M.S. technology17,18, or orthokeratology, as reported by Dr. Pauline Cho, with a 43% myopia control rate compared to single-vision glasses19. However, participants in that study still had positive annual AL value changes.
The mechanism underlying the negative values of changes in AL is unclear since there were no other parameters studied herein, such as SFChT, ACD, or CCP, that could reasonably explain this phenomenon.
The first RCT with a 6-month follow-up on PBM intervention in children with myopia reported a statistically significant thicker SFChT3, while our results revealed no statistically significant difference between the two groups due to the accuracy of OCT choroidal thickness and the limited sample size. According to our experimental data, 65% of the individuals with increased SFChT in the PBM group reported a decreased AL at the last visit, while only 36% of the individuals with thinner SFChT in the control group reported an increase in AL during the same period. It remains unclear whether the changes in SFChT can explain the changes in AL because PBM therapy was defined as the utilization of nonionizing electromagnetic energy to trigger photochemical changes within cellular structures that absorbed photos. Mitochondria are particularly sensitive to this process, with more ATP for cellular use with a key enzyme called cytochrome oxidase C. In addition, that process produces mild oxidants (ROS), leading to gene transcription, which then leads to cell repair and healing. This process can also remove the chains clogged by nitric oxide (NO).
NO is a molecule produced by our body that helps the 50 trillion cells in our body communicate with each other through signalling, helping to dilate blood vessels, and improving blood circulation. Our vision is based on light hitting our retina and generating a chemical reaction that enables us to see.
Regarding vision, none of the participants had reduced BCVA. BCVA was the inclusive criterion for enrolment in the current study, and there was no special record to compare the differences between the two groups. However, some studies have reported significantly better vision after PBM therapy5.
In view of the limitations of this study, a non-double-blind study was designed. We did not provide sham devices for the control group, thus avoiding the subjective tendency of the treatment or placebo effect. Additionally, there was a high withdrawal rate at baseline in the control group after randomization, which disrupted the balance in the ages of the two groups. The significant bias created by having a significantly younger control group at baseline. The mean age of the PBM group (mean age: 10.2 ± 1.6 years) was older than that of the control group (mean age: 9.0 ± 1.2 years), and it was known that axial length increases faster in younger people. Although at 9-month visit, the mean ages from the two groups (9.9 ± 1.4 years, vs. 8.4 ± 0.5 years) were not statistically significant (P = 0.07).
Another limitation was the limited sample size and the slightly high dropout rate, especially in the control group. The limited number of samples might have explained the lack of a significant difference in SFChT or ACD, since the calculation of sample number was based on the AL only.
Future research should explore the different sources of indoor light, such as light emitting diode (LED), or related parameters, such as laser output energy, radiation wavelength, exposure duration and laser beam cross-sectional area of the point of interest. Additionally, different forms of administration in the clinic should be examined.
There is still a long way to go to determine the optimal phototherapy parameters for myopia or other related diseases. Long-term prospective programs are needed to identify the optimal conditions. In addition, the long-term effect of this technology remains unclear.
Briefly, the current 12-month study showed that PBM is a new, noninvasive and safe intervention with higher efficacy and safety for controlling, delaying and reversing the progression of myopia in children.
Conclusions
PBM therapy is an effective treatment to delay and retard increases in AL among children with myopia; this therapy is accompanied by approximately 2.1 min of temporary vision loss.
Data availability
Datasets of this study are available from the corresponding authors upon reasonable request.
References
-
Wu, P. C. et al. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology 125, 1239–1250 (2018).
-
Smith, E. L. 3rd., Huang, L. F. & Huang, J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Investig. Ophthalmol. Vis. Sci. 53, 421–428 (2012).
-
Xiong, F. et al. Orthokeratology and low-intensity laser therapy for slowing the progression of myopia in children. Biomed. Res. Int. 1, 8915867. https://doi.org/10.1155/8915867 (2021).
-
Zhou, L., Xing, C., Wei, Q., Hua, C. & Tong, L. Low-intensity, long-wavelength red light slows the progression of myopia in children: An Eastern China-based Cohort. Ophthalmic Physiol. Opt. 42, 335–344 (2022).
-
Jiang, Y. et al. Effect of repeated low-level red-light therapy in myopia control in children: A multicenter randomized controlled trial. Ophthalmology 129, 509–519 (2022).
-
Dong, J., Zhu, Z., Xu, H. & He, M. Myopia control effect of repeated low-level red-light therapy in Chinese children: A randomized, double-blind, controlled clinical trial. Ophthalmology 130, 198–204 (2023).
-
Xiong, R. et al. Sustained and rebound effect of repeated low-level red-light therapy on myopia control: A 2-year post-trial follow-up study. Clin. Exp. Ophthalmol. 50, 1013–1024 (2022).
-
Chen, H. et al. Low-intensity red-light therapy in slowing myopic progression and the rebound effect after its cessation in Chinese children: A randomized controlled trial. Graefes Arch. Clin. Exp. Ophthalmol. 261, 575–584 (2023).
-
Zhao, Y., et al. The ocular axis length was shortened by 1.03mm with decreased myopic refraction of −3.00D. Don’t believe myopia cannot be treated. WeChat. https://mp.weixin.qq.com/s/1_c_omBDYnTGnXphEdHhUw (2022). https://mp.weixin.qq.com/s/N6FIBwVizHntd4L62Ow81g (2022).
-
Stone, R. A. et al. Diurnal axial length fluctuations in human eyes. Investig. Ophthalmol. Vis. Sci. 45, 63–70 (2004).
-
Yi, S. et al. Therapeutic effect of atropine 1% in children with low myopia. J. AAPOS 19, 426–429 (2015).
-
Chia, A. et al. Atropine for the treatment of childhood myopia: Safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2). Ophthalmology 119, 347–354 (2012).
-
Ruiz-Pomeda, A. et al. MiSight assessment study Spain (MASS) a 2-year randomized clinical trial. Graefes Arch. Clin. Exp. Ophthalmol. 256, 1011–1021 (2018).
-
Chamberlain, P. et al. A 3-year randomized clinical trial of MiSight lenses for myopia control. Optom. Vis. Sci. 96, 556–567 (2019).
-
Yam, J. C. et al. Low-concentration atropine for myopia progression (LAMP) study: A randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology 126, 113–124 (2019).
-
Yam, J. C. et al. Two-year clinical trial of the low-concentration atropine for myopia progression (LAMP) study: Phase 2 report. Ophthalmology 127, 910–919 (2020).
-
Lam, C. S. et al. Defocus incorporated multiple segments (DIMS) spectacle lenses slow myopia progression: A 2-year randomized clinical trial. Br. J. Ophthalmol. 104, 363–368 (2020).
-
Lam, C. S. et al. Myopia control effect of defocus incorporated multiple segments (DIMS) spectacle lens in Chinese children: Results of a 3-year follow-up study. Br. J. Ophthalmol. 106, 1110–1114 (2022).
-
Cho, P. et al. Retardation of myopia in orthokeratology (ROMIO) study: A 2-year randomized clinical trial. Investig. Ophthalmol. Vis. Sci. 53, 7077–7085 (2012).
-
Wang, W. et al. Clinically significant axial shortening in myopic children after repeated low-lever red light therapy: A retrospective multicenter analysis. Ophthalmol. Ther. 1, 2. https://doi.org/10.1007/s40123-022-00644-2 (2023).
-
Chen, Y. et al. Efficacy comparison of repeated low-level red light and low-dose atropine for myopia control: A randomized controlled trial. Transl. Vis. Sci. Technol. 11, 33 (2022).
Acknowledgements
The authors thank the corporation of LONGDA for providing the photobiomodulation therapy devices. In addition, the authors gratefully acknowledge the ophthalmic technicians who helped conduct this trial at Ningbo Eye Hospital. Additionally, the authors appreciate the support from the South East Eye Hospital in Fuzhou. Finally, the authors sincerely thank the families and children who participated in this study.
Funding
Supported by a grant from the Science and Technology Program of Ningbo, China (grant no. 2020Y55) and a grant from Xuzhou Medical University affiliated with Xuzhou Municipal Hospital Development Grant no. XYFM2020028.
Author information
Authors and Affiliations
-
Department of Optometry, Ningbo Eye Hospital, Ningbo, 315000, Zhejiang Province, China
Lei Zhou & Liyang Tong
-
Department of Ophthalmology, The First People’s Hospital of Xuzhou, The Affiliated Xuzhou Municipal Hospital of Xuzhou Medical University, Xuzhou, 221005, Jiangsu Province, China
Ying Li
-
Xuzhou Medical University, Xuzhou, 221005, Jiangsu Province, China
Ying Li
-
Seattle Vision Care Center, Seattle, WA, 98101, USA
Bruce T. Williams
-
Tianming Ophthalmology and Optometry Clinics, Dali, 671099, Yunnan, China
Kaikai Qiu
-
Airdoc MPC Co, Ltd, F4, No.2 North Road, Haidian District, Beijing, 100081, China
Kaikai Qiu
Contributions
L.Z. and K.Q. conceived the research ideas. L.Z. and L.T. performed the experiments. K.Q. analyzed the data and wrote the main manuscript text. Y.L. and T.L. provided the related references and funding. W.B. ran a thorough check on the use of English for scientific writing. All authors contributed to manuscript revision, and each read and approved the revision and final manuscript. All study subjects provided informed consent.
Corresponding author
Correspondence to Kaikai Qiu.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About Passiva
Passiva is an innovative materials science company that provides a revolutionary biotech platform to cost-effectively bring device-free therapeutic red light capabilities as an added ingredient or component to everyday medical, cosmetic, or vision care products.